Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- XFEL Pulses Demonstrate How Plants Perceive Light
- Structural biology is solved -- now what?
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
- Details
- Monday, 18 May 2015

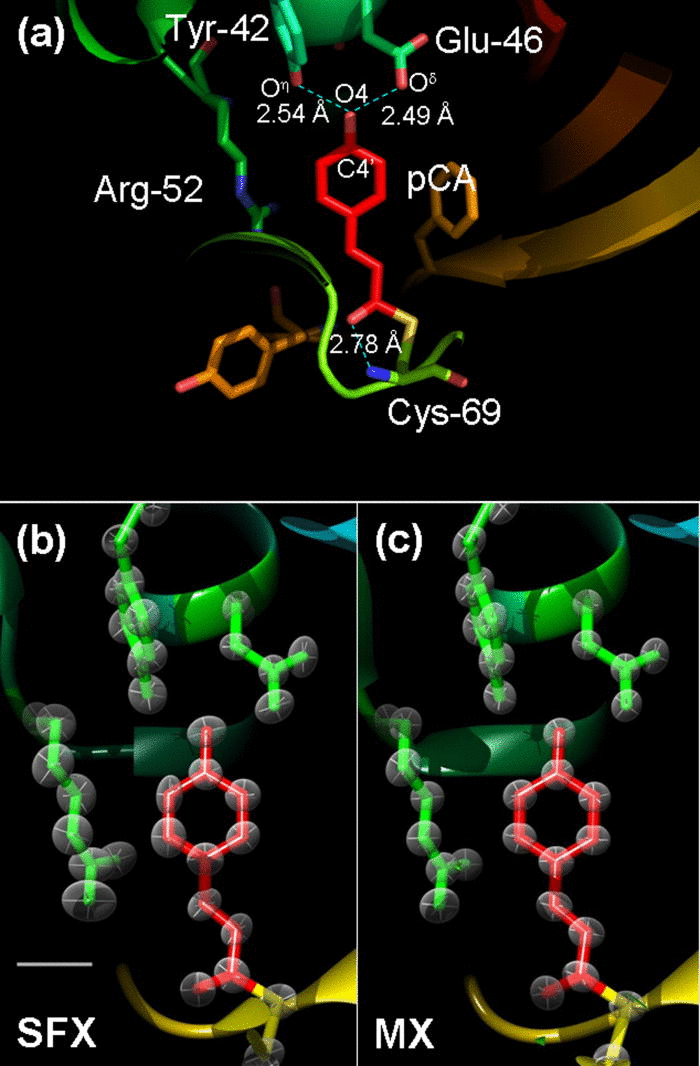
We push the resolution of macromolecular serial femtosecond crystallography to 1.46 Å. Anisotropic B-factor refinement starts to become possible with SFX data at that resolution. The structures show little to no evidence of radiation damage even at ambient temperatures and are comparable to ultrahigh (atomic) resolution structures.
Recent BioXFEL publication in Structural Dynamics by Marius Schmidt, Kanupriya Pande, Shibom Basu, and Jason Tenboer.
See full article here.
About 2.5 × 106 snapshots on microcrystals of photoactive yellow protein (PYP) from a recent serial femtosecond crystallographic (SFX) experiment were reanalyzed to maximum resolution. The resolution is pushed to 1.46 Å, and a PYP model is refined at that resolution. The result is compared to other PYP models determined at atomic resolution around 1 Å and better at the synchrotron. By comparing subtleties such as individual isotropic temperature factors and hydrogen bond lengths, we were able to assess the quality of the SFX data at that resolution. We also show that the determination of anisotropic temperature factor ellipsoids starts to become feasible with the SFX data at resolutions better than 1.5 Å.





