Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- Structural biology is solved -- now what?
- XFEL Pulses Demonstrate How Plants Perceive Light
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
- Details
- Thursday, 24 August 2017

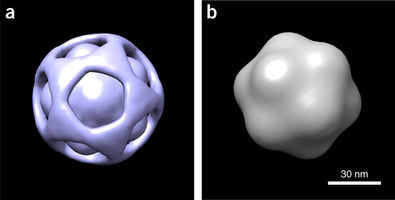
BioXFEL researcher Peter Schwander released a publication, along with his peers, to Nature Methods
Using a manifold-based analysis of experimental diffraction snapshots from an X-ray free electron laser, we determine the three-dimensional structure and conformational landscape of the PR772 virus to a detector-limited resolution of 9 nm. Our results indicate that a single conformational coordinate controls reorganization of the genome, growth of a tubular structure from a portal vertex and release of the genome.
These results demonstrate that single-particle X-ray scattering has the potential to shed light on key biological processes.
Almost 20 years ago, pioneering simulations by Neutze et al. showed that damage-free imaging of individual biological entities, first proposed by Breedlove and Trammell, and later by Solem and Baldwin, may indeed be possible with X-ray free electron lasers (XFELs). This 'single-particle imaging' approach involves recovering three-dimensional (3D) structure from a collection of 2D snapshots of individual particles, each viewed in an unknown orientation. The 3D structures of large viruses have been determined with this approach, albeit to resolutions substantially lower than expected from the highest scattering vector recorded on the detector.





