Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- XFEL Pulses Demonstrate How Plants Perceive Light
- Structural biology is solved -- now what?
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
- Details
- Monday, 13 September 2021

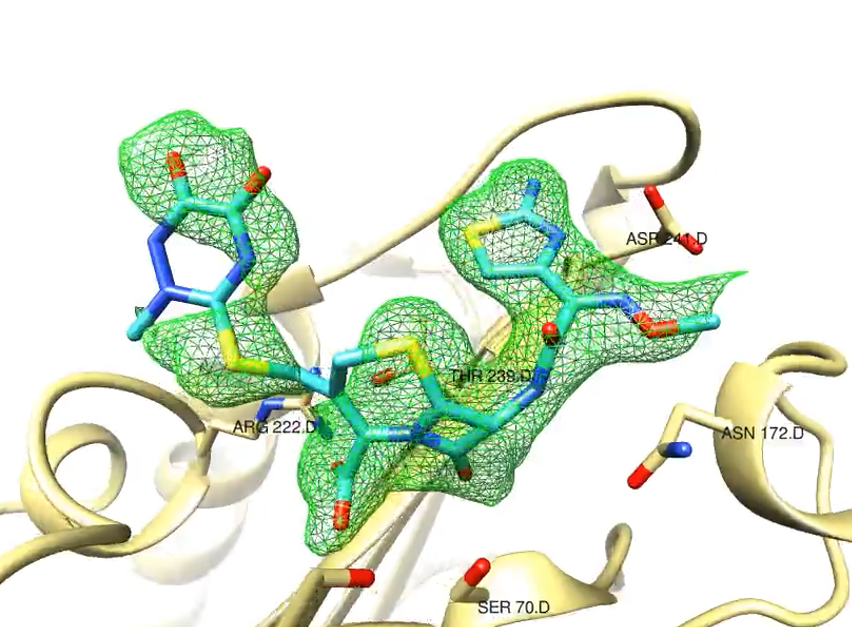
Using the X-ray laser European XFEL an international team of researchers has successfully filmed a reaction step that is important for the development of antibiotic resistance.
The molecular film captures the very rapid reaction of the enzyme beta-lactamase from tuberculosis bacteria with the cephalosporin antibiotic ceftriaxone in slow motion. The team published the results in IUCrJ, the journal of the International Union of Crystallography.
Antibiotic resistance occurs, in part, when bacteria acquire the ability to inactivate the antibiotics used against them. Many resistant bacteria produce the enzyme beta-lactamase. This enzyme can render the most commonly used beta-lactam antibiotics ineffective. The team, led by Marius Schmidt of the University of Wisconsin-Milwaukee, have now studied the first step of this inactivation at the SPB/SFX experimental station of the European XFEL, observing how the antibiotic binds to the beta-lactamase from resistant tuberculosis bacteria within milliseconds (thousandths of a second). The researchers also investigated how the enzyme inhibitor sulbactam reacts with the bacterial enzyme. Sulbactam, administered at the same time as the antibiotics, binds to the bacterial beta-lactamase, thereby blocking it. As a result the enzyme is unable to inactivate the antibiotics whose effectiveness is restored this way.
Up to now, crystals have been a requirement to study biomolecules using X-rays, but with the advent of free electron X-ray lasers, researchers are able to study extremely small crystals. In the case of beta-lactamase, the enzyme crystals and the antibiotic solution are mixed together very quickly, and the reaction starts when the antibiotic diffuses into the enzyme crystal. Because of the small size of the crystals, diffusion is very fast, and all beta-lactamase molecules in the crystal react with the antibiotic solution almost simultaneously. The special mix-and-inject method used in the study requires custom-made injectors, which were provided by the group of Lois Pollack (Cornell University) for use at the European XFEL. The method enables atomically accurate slow-motion imaging of fast biological processes in enzymes and other biomolecules.
"Enzymes such as beta-lactamase are of great importance for medicine," explains Marius Schmidt. "X-ray lasers like the European XFEL now make it possible to study much smaller crystals than in the past. Our experiment, together with previous results, has shown how X-ray lasers can be used as an important tool for biological research in the future," he adds.
The paper can be accessed under doi.org/10.1107/S2052252521008125.
Modified from a press-release by Bernd Ebeling at the European XFEL.





