Popular Articles
- Earliest molecular events of vision revealed
- Dynamics and Kinetics in Structural Biology
- Structural biology is solved -- now what?
- XFEL Pulses Demonstrate How Plants Perceive Light
- BioXFEL Postdoctoral Fellowship Award
Archived Articles
News
- Details
- Tuesday, 29 August 2017
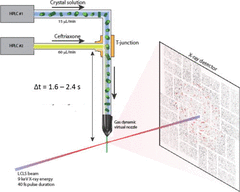
Mix-and-inject serial crystallography (MISC) is a technique designed to image enzyme catalyzed reactions in which small protein crystals are mixed with a substrate just prior to being probed by an X-ray pulse. This approach offers several advantages over flow cell studies. It provides (i) room temperature structures at near atomic resolution, (ii) time resolution ranging from microseconds to seconds, and (iii) convenient reaction initiation.
- Details
- Thursday, 24 August 2017
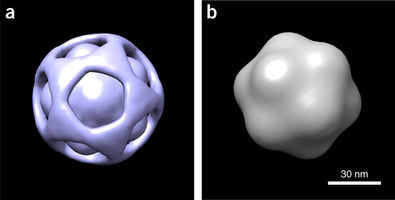
BioXFEL researcher Peter Schwander released a publication, along with his peers, to Nature Methods
Using a manifold-based analysis of experimental diffraction snapshots from an X-ray free electron laser, we determine the three-dimensional structure and conformational landscape of the PR772 virus to a detector-limited resolution of 9 nm. Our results indicate that a single conformational coordinate controls reorganization of the genome, growth of a tubular structure from a portal vertex and release of the genome.
- Details
- Monday, 14 August 2017
A research collaboration led by the University of Wisconsin-Milwaukee has for the first time created a three-dimensional movie showing a virus preparing to infect a healthy cell.
The research has the potential to fundamentally advance our understanding of how biological processes inside the cell work. That could lead to better treatment for the horde of human diseases caused by viruses.
The feat was made possible by UWM physicists, who developed a new generation of powerful algorithms to reconstruct sequential images from an ocean of unsorted, noisy data.
- Details
- Thursday, 10 August 2017
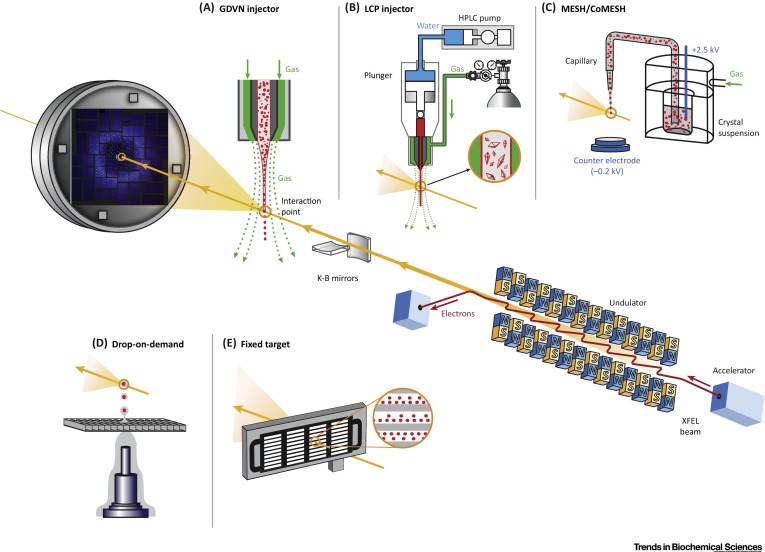
BioXFEL scientist Vadim Cherezov and his colleagues released a new publication to CellPress:
X-ray free electron lasers (XFELs) have the potential to revolutionize macromolecular structural biology due to the unique combination of spatial coherence, extreme peak brilliance, and short duration of X-ray pulses. A recently emerged serial femtosecond (fs) crystallography (SFX) approach using XFEL radiation overcomes some of the biggest hurdles of traditional crystallography related to radiation damage through the diffraction-before-destruction principle.
- Details
- Tuesday, 08 August 2017
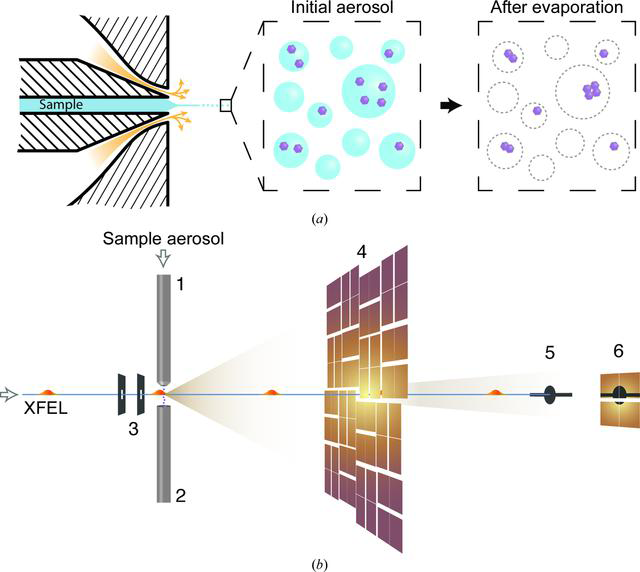
BioXFEL scientist Richard A. Kirian, along with his peers, published a research article onto IUCrj:
This study explores the capabilities of the Coherent X-ray Imaging Instrument at the Linac Coherent Light Source to image small biological samples. The weak signal from small samples puts a significant demand on the experiment. Aerosolized Omono River virus particles of ∼40 nm in diameter were injected into the submicrometre X-ray focus at a reduced pressure.
- Details
- Thursday, 03 August 2017
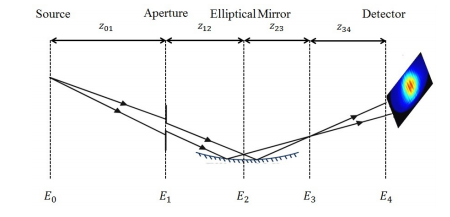
BioXFEL scientist Marc Messerschmidt, along with his colleagues, published a research article in OSA publishing.
Summary:
Determining fluctuations in focus properties is essential for many experiments at Self-Amplified-Spontaneous-Emission (SASE) based Free-Electron-Lasers (FELs), in particular for imaging single non-crystalline biological particles.
More Articles...
- Critical Role of Water Molecules in Proton Translocation by the Membrane-Bound Transhydrogenase
- Structural insights into the extracellular recognition of the human serotonin 2B receptor by an antibody
- FELIX: an algorithm for indexing multiple crystallites in X-ray free-electron laser snapshot diffraction images
- Crystal structure of CO-bound cytochrome c oxidase determined by serial femtosecond X-ray crystallography at room temperature
- New algorithms extract biological structure from limited data





